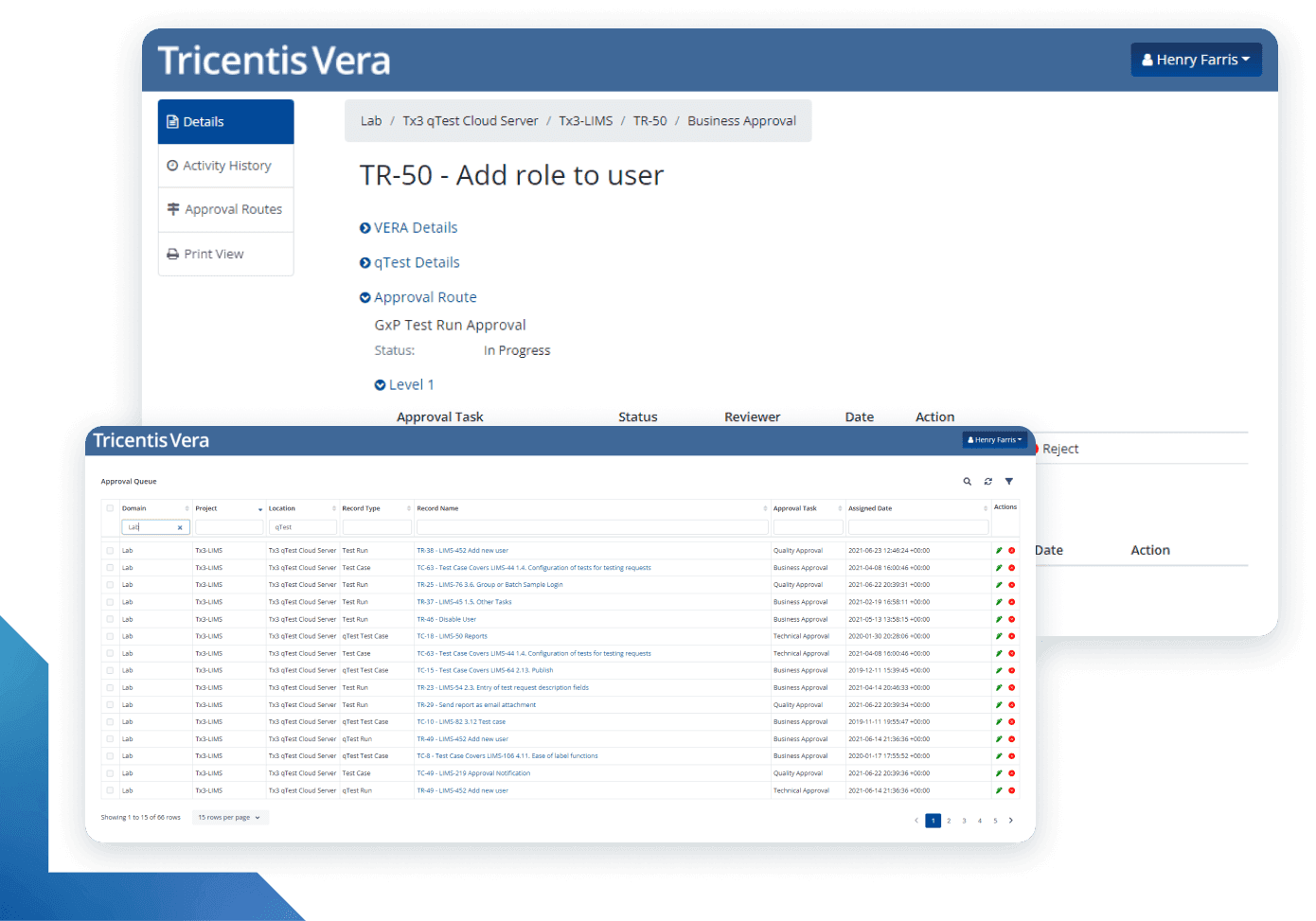
More than just electronic signatures
With Tricentis Vera, Digital Validation means more than just applying electronic signatures to shift traditional documents to a computer screen. It means modernizing the Computer Systems Validation process to shed cumbersome, extraneous documentation practices and enabling compliance to be achieved as a byproduct of good software quality practices.